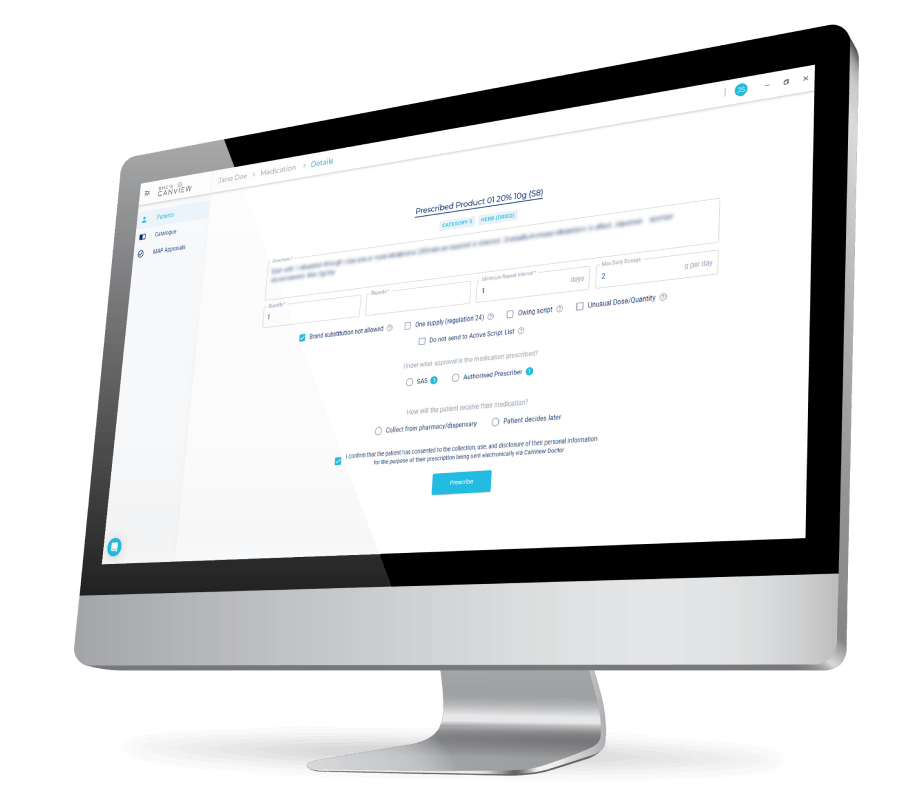
SAS B allows prescribers (including nurse practitioners) to prescribe medicinal cannabis products for a single patient on a case-by-case basis. Special Access Scheme Category B (SAS B) is an application pathway through which a registered health practitioner may apply to the TGA for approval to prescribe an unapproved medicinal cannabis product for a patient under their care. The applicant must provide a suitable clinical justification for the use of the therapeutic good, including reasons why products included in the ARTG are not suitable for treating the patient.
Authorised Prescriber. Any Australian registered medical practitioner can become an AP. The TGA can grant a medical practitioner authority to prescribe a specified unapproved medicinal cannabis product for particular indications to a class of patients in their immediate care. To become an AP, a medical practitioner must obtain approval from a Human Research Ethics Committee (HREC) or endorsement from an appropriate specialist college to prescribe the product. Prescribers do not need HREC approval or specialist college endorsement if the selected medicine’s active ingredient category, dosage form and indication are included in the TGA’s List of medicinal cannabis medicines with an established history of use. APs do not need to notify the TGA each time they prescribe medicinal cannabis products during the approval period (up to 5 years). APs must report the number of patients they treat every 6 months, even if the number is zero.